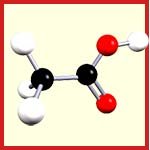
Chemistry only
Alcohols are flammable; they make excellent fuels. Since
alcohols contain only the elements carbon,
hydrogen and oxygen and combustion simply involves adding oxygen to the elements present in a
compound then combustion of alcohols will release carbon dioxide and water vapour:
e.g 1: the equations below show the combustion of the alcohol methanol:

The smaller the alcohol molecule the more volatile it will be and so the more flammable it will be.
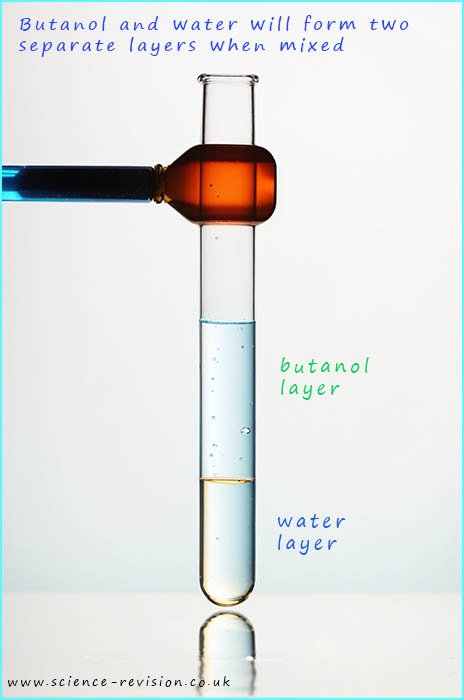
Alcohols are generally soluble in water however as the alcohol molecules get larger (chain length increases) the solubility drops. While the alcohols methanol and ethanol are all miscible in water, that is they can be mixed in any ratio without separating out into two separate phases or layers, as oil and water do. However as the chain length increases the solubility of the alcohol molecule in water begins to fall.
Propanol will dissolve in water but butanol is barely soluble in water. If a small amount of butanol is added to water it will dissolve in the water but as more is added then the butanol will begin to form its own separate layer which floats on top of the water. This is shown in the image opposite. Alcohols which do dissolve in water will form neutral solutions with a pH of 7.
Ethanol is the only alcohol which is found in alcoholic drinks; this is simply because it is the only alcohol that is safe to consume in small amounts and it is a highly effective intoxicant; that is it causes the desired effects of relaxation and euphoria.

Methanol is highly toxic and can cause blindness or death even in small quantities. Propanol and butanol are less toxic than methanol but have unpleasant tastes and smells, making them very unpleasant to consume; they also act as depressants and irritants and cannot be easily processed by the liver into carbon dioxide and water like ethanol.
The reaction of sodium metal with alcohols is very similar to that with water. At first glance this may
seem odd as alcohols and water molecules appear to be very different from each other.
However all the parts of the alcohols circled in the image below do not take part in any of the chemical reactions of the
alcohol molecules;
only the reactive hydroxyl functional group (R-OH) group on the alcohol takes part in its chemical reactions (other than combustion).
The parts of the alcohol molecules circled in red in the image below are called alkyl groups. An alkyl group is just the alcohol molecule without the reactive hydroxyl functional group (-OH) . For example:
Alkyl groups are often shown as the letter R in chemical formulas. This is because they are mostly unreactive and don’t usually take part in the chemical reactions of the alcohols. The reactions mainly happen at the hydroxyl group (-OH), so alcohols are often written as R–OH.
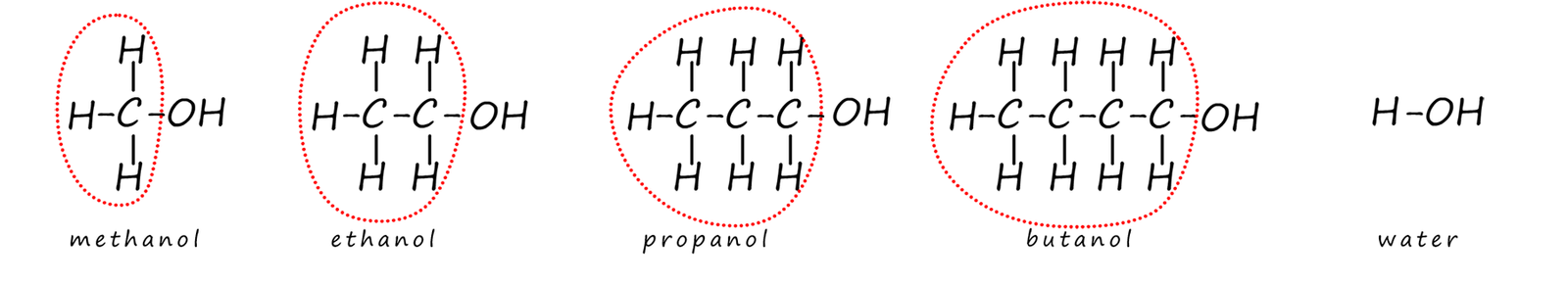
So if you swap all these circles groups and simply replace them with a -R label to represent them, then we have:

Remember the reaction of sodium with water:
Well the hydroxide ion (OH-) in the sodium hydroxide is simply a water molecule (H2O), or H-OH which has lost a hydrogen ion; this lost hydrogen atoms are given off as a molecule of hydrogen gas during the reaction.
When sodium reacts with alcohols the same reaction occurs. The sodium removes the hydrogen attached to the hydroxyl functional group in the alcohol. The lost hydrogen is given off as hydrogen gas.
As mentioned above the structure of an alcohol can be considered in some ways similar to that of a water molecule. However as the chain length of the alcohol grows then the reactions of the alcohol with sodium metal begins to slow down. Methanol (CH3OH) is the "smallest" alcohol and it reacts the fastest of all the alcohols with sodium metal. Ethanol (C2H5OH) the next alcohol reacts more slowly with sodium and propanol the next alcohol in the homologous series reacts even more slowly. This is shown in the image below where the rate of release of hydrogen gas gives an excellent indicator of the speed of the reaction taking place.

When a substance is oxidised oxygen is added to it or hydrogen is removed. This is one definition of oxidation that I am sure you are familiar with. If wine which contains the alcohol ethanol is left exposed to air it will go off. The ethanol in the wine will react with oxygen in the air to give it a sour taste. The reason the wine tastes sour is that the ethanol has been oxidised into ethanoic acid; a carboxylic acid. The oxidation of the alcohol ethanol to ethanoic acid is shown in the image below where the oxidising agent is represented by the symbol [O]:
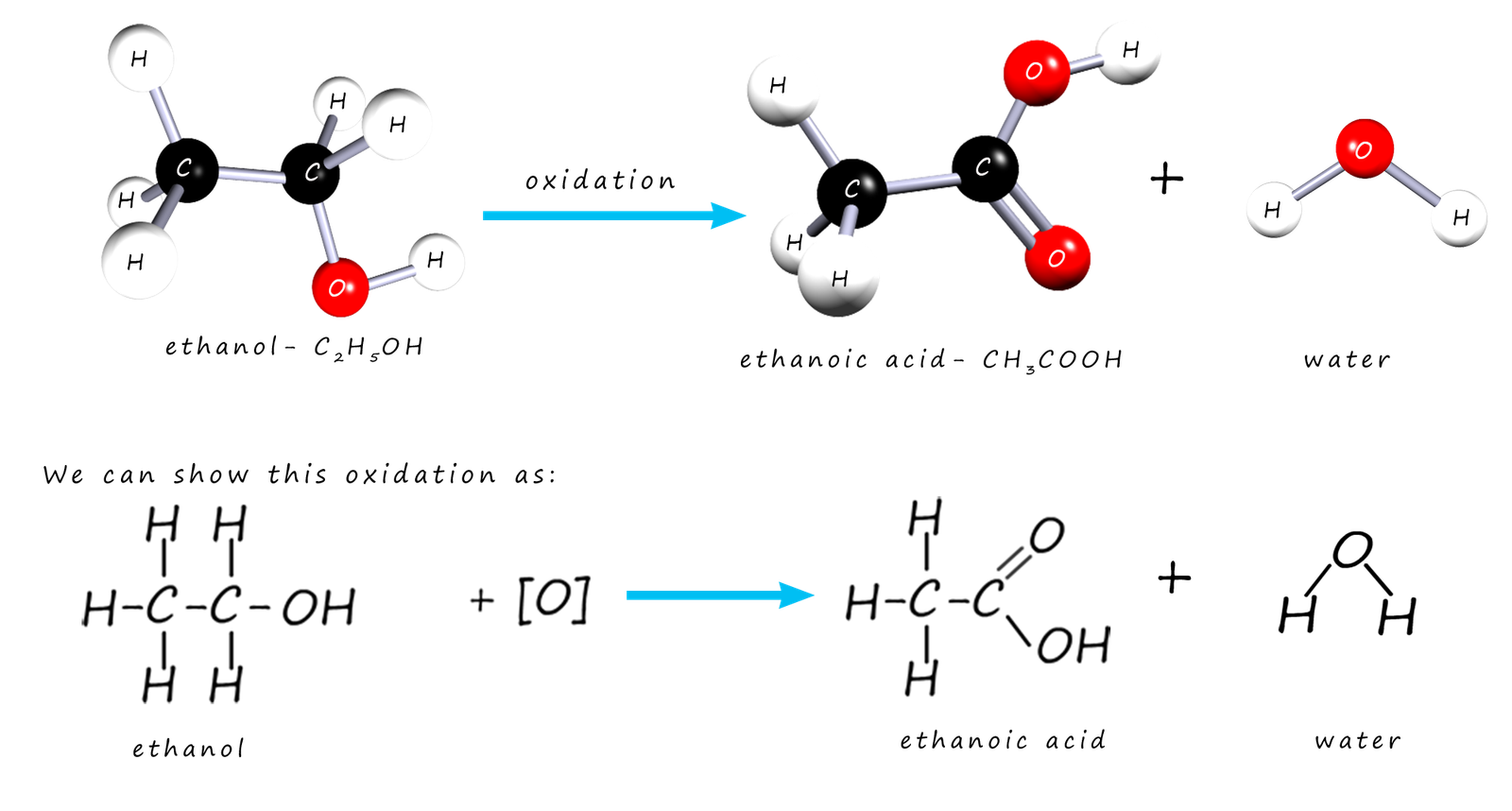
 If you study the diagrams above you will see that alcohol ethanol has an oxygen atom added and 2 hydrogen atoms removed from it when it is
oxidised to ethanoic acid. The alcohol has been oxidised to a carboxylic acid. The apparatus diagram on the right gives
an outline on how this oxidation reaction is carried out in the lab.
If you study the diagrams above you will see that alcohol ethanol has an oxygen atom added and 2 hydrogen atoms removed from it when it is
oxidised to ethanoic acid. The alcohol has been oxidised to a carboxylic acid. The apparatus diagram on the right gives
an outline on how this oxidation reaction is carried out in the lab.
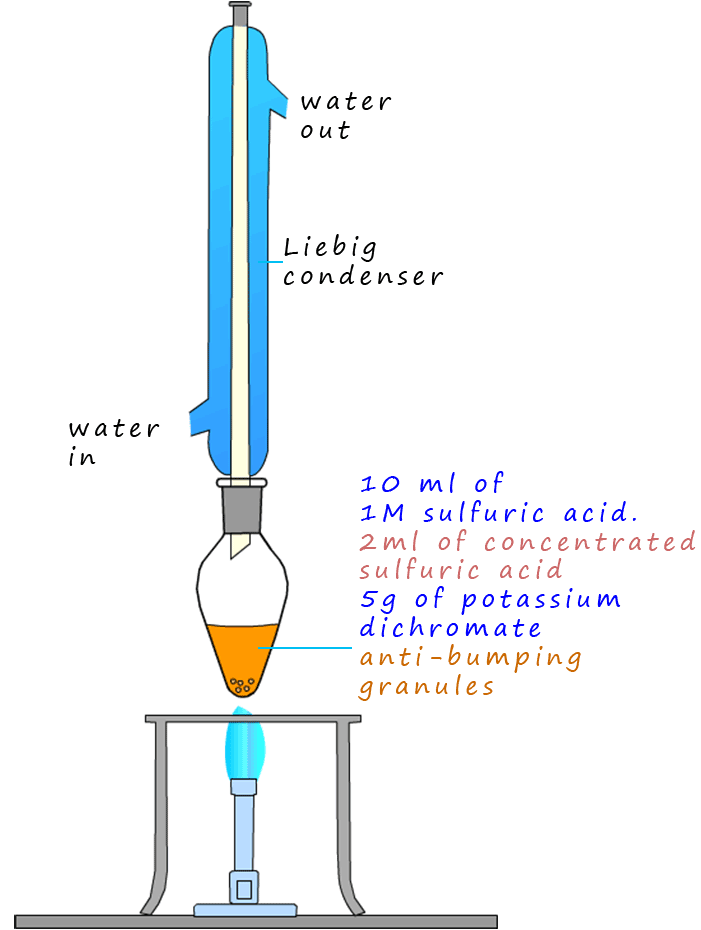
The set-up shown opposite where the Liebig condenser is attached directly on top of the pear shaped flask is called reflux.
The oxidising agent is a bright orange solid compound called potassium dichromate.
Potassium dichromate is a particularly unpleasant compound; it is
a well known carcinogen and to make matters worse it is dissolved in dilute sulfuric acid, a corrosive solution, then a few mls of concentrated
sulfuric acid is added to the mixture as well! However this acidified potassium dichromate
solution is an excellent
 oxidising agent.
The ethanol is simply poured very slowly down the Liebig condenser and into the pear shaped flask containing the oxidising mixture and then heated for around 20 minutes.
oxidising agent.
The ethanol is simply poured very slowly down the Liebig condenser and into the pear shaped flask containing the oxidising mixture and then heated for around 20 minutes.
The condenser is inserted vertically into the pear shaped flask containing the mixture of chemicals because the ethanol and
other intermediate compounds produced are volatile and would simply evaporate out of the flask before the acidified
potassium dichromate solution has a chance to oxidise them fully. This way when the volatile substances evaporate, they are cooled and
condense inside the Liebig condenser and drip back into the pear shaped flask to be oxidised further.
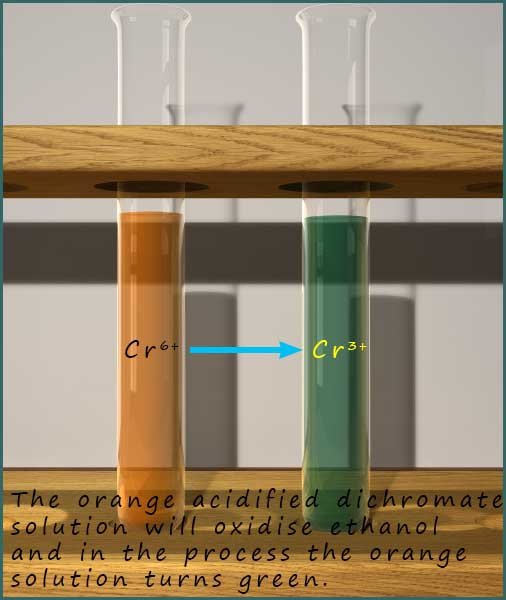
The dichromate ion has the formula Cr2O72-, it contains chromium ions with a +6 charge (Cr+6). It is the presence of these ions which gives the dichromate solution its distinctive orange colour. However when the acidified potassium dichromate solution oxidises ethanol then the orange chromate ions (Cr6+) gain 3 electrons and are reduced to form the green chromium(III) ion (Cr3+). This is shown in the image opposite.
Use the flashcards below to review your understanding of the chemistry involved when alcohols are oxidised.